Abstract

video camera. See video below.
A wire glows orange while submerged in water.
Portable
Yes
Principles Illustrated
Transfer of thermal energy. Thermal conductivity. Energy transformations.
The wire can be hot enough to glow because it is surrounded by a vapour layer. When the current is turned off, the water reaches the wire and a hiss is heard as the wire cools rapidly. A similar effect can be observed when an object is placed in liquid nitrogen and a vapour layer forms. The boiling is slow until the object cools somewhat, and then the liquid reaches the object the boiling suddenly increases while the object is cooled to liquid nitrogen temperature.
Video
Download video (right-click and “save link as”): Cooling-wire.m4v
NCEA & Science Curriculum
Can be used as a starting point for investigations in PHYS 1.1, PHYS 1.2.
Instructions
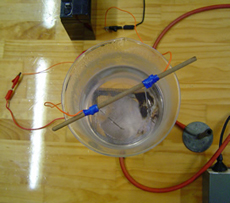
electrical connections.
Boil the water with a flame. Connect the current source (here a battery) to the nichrome wire and observe. Some adjustment of the current and wire length will be necessary.
Larger photos
Beaker and electrical connections
Safety
Use only a low voltage, current-limited power supply or battery! There is a fairly large quantity of boiling water and a flame involved. Constant supervision is necessary.
Individual teachers are responsible for safety in their own classes. Even familiar demonstrations should be practised and safety-checked by individual teachers before they are used in a classroom.
Related Resources
Teaching Resources
Would you like to contribute lesson suggestions? Contact us.
Credits
This teaching resource was developed with support from
The MacDiarmid Institute
Faculty of Science, Victoria University of Wellington
School of Chemical and Physical Sciences, Victoria University of Wellington